
The FDA final ruling to improve access to hearing aids will not impact only Americans. The highly anticipated documents and guidance intended to "improve access to safe, effective, and affordable hearing aids" will define market behavior globally by determining how manufacturers will approach the market. For traditional hearing aid companies, this is the opportunity they have been waiting for launching new-generation products that will expand their market considerably - before the market is flooded with smart earbuds controlled by apps.
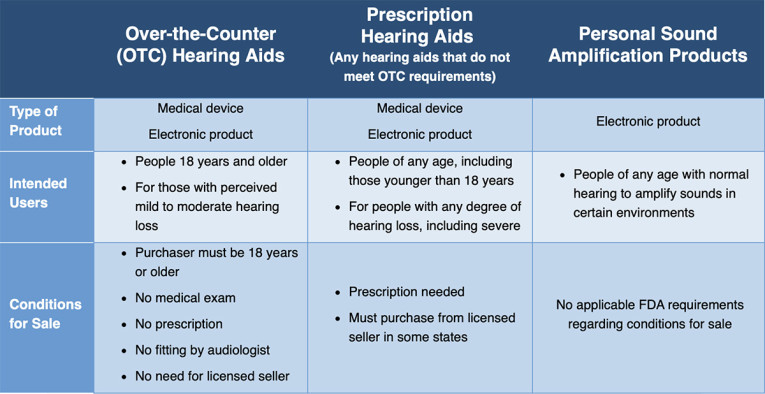
The FDA rule establishes a new category of over-the-counter (OTC) hearing aids, enabling consumers with perceived mild to moderate hearing impairment to purchase hearing aids directly from stores or online retailers without the need for a medical exam, prescription or a fitting adjustment by an audiologist.
The rule is expected to lower the cost of hearings aids, furthering the Biden-Harris Administration’s goal of expanding access to high-quality health care and lowering health care costs for the American public. It is designed to assure the safety and effectiveness of OTC hearing aids, while fostering innovation and competition in the hearing aid technology marketplace.
The release of the final rule and guidance follows President Biden's Executive Order on Promoting Competition in the American Economy, which called for the FDA to take steps to allow hearing aids to be sold over the counter and set a swift 120-day deadline for action, which the FDA met. In 2017, Congress passed bipartisan legislation requiring the FDA to create a category of OTC hearing aids, but it was not fully implemented until now. Consumers could see OTC hearing aids available in traditional retail and drug stores as soon as mid-October when the rule takes effect.
Close to 30 million adults in the US could benefit from hearing aid use. Individuals with permanent hearing impairment can use hearing aids to help make speech and sounds louder, improving the ability to communicate effectively with others. Many hearing aids can be expensive. The final rule aims to stimulate competition and facilitate the sale of safe and effective OTC hearing aids in traditional retail stores or online nationwide, providing consumers with perceived mild to moderate hearing loss with improved access to devices that meet their needs and are less expensive than current options.

"Hearing loss is a critical public health issue that affects the ability of millions of Americans to effectively communicate in their daily social interactions," says FDA Commissioner Robert M. Califf, M.D. "Establishing this new regulatory category will allow people with perceived mild to moderate hearing loss to have convenient access to an array of safe, effective and affordable hearing aids from their neighborhood store or online."
The OTC category established in this final rule applies to certain air-conduction hearing aids intended for people 18 years of age and older who have perceived mild to moderate hearing impairment. Hearing aids that do not meet the requirements for the OTC category (for example, because they are intended for severe hearing impairment or users younger than age 18) remain prescription devices.
Comments Addressed
The FDA finalized the rule after receiving and reviewing more than 1,000 public comments on the proposed rule issued on Oct. 20, 2021. Comments submitted by consumers, professional associations, hearing aid manufacturers, public health organizations and advocacy groups, members of Congress, state agencies, and other stakeholders are summarized in the final rule, along with the FDA’s respective responses.
In response to public comments and to assure the safety and effectiveness of OTC hearing aids, the final rule incorporates several changes from the proposed rule, including lowering the maximum sound output to reduce the risk to hearing from over-amplification of sound, revising the insertion depth limit in the ear canal, requiring that all OTC hearing aids have a user-adjustable volume control, and simplifying the phrasing throughout the required device labeling to ensure it is easily understood. The final rule also includes performance specifications and device design requirements specific to OTC hearing aids.
Furthermore, the new documents amend existing rules that apply to prescription hearing aids for consistency with the new OTC category, it repeals the conditions for sale for hearing aids, and it includes provisions that address some of the effects of the FDA OTC hearing aid regulations on state regulation of hearing aids.

Concurrently with issuing the final rule, the FDA also issued the final guidance, Regulatory Requirements for Hearing Aid Devices and Personal Sound Amplification Products (PSAPs), to clarify the differences between hearing aids, which are medical devices, and PSAPs, consumer products that help people with normal hearing amplify sounds.
The effective date for the final rule is 60 days following publication in the Federal Register. Manufacturers of hearing aids sold prior to the effective date of the final rule will have 240 days after its publication to comply with the new or revised requirements. For hearing aids that have not been offered for sale prior to the effective date, compliance with the new or revised requirements must be achieved before marketing the device, including obtaining 510(k) clearance if applicable.
HIA Support
The Hearing Industries Association (HIA), which represents hearing aid manufacturers, suppliers, distributors, and hearing health professionals, published a statement strongly supporting the FDA OTC rule.
"This is a significant step forward for the millions of Americans who suffer hearing loss yet are untreated," says Kate Carr, President of HIA. "Hearing loss is unique to each person, and most do not know if their condition is mild, moderate, or greater, caused by another medical issue or something as simple as ear wax. HIA supports the final rule and recommends that the best treatment for hearing loss involves seeing a hearing professional."
Earlier in 2022, the HIA, in partnership with multiple industry leaders, announced the Hear Well campaign, aiming to educate the public about the importance of their hearing health and the value of seeing a hearing professional. The "Hear Well" campaign is educating audiences through an integrated, multi-platform approach using social media, traditional media, and paid advertisements.
The campaign is sponsored by the Hearing Industries Association and its members. Other campaign partners joining the effort include Academy of Doctors of Audiology, Alexander Graham Bell Association for the Deaf and Hard of Hearing, American Academy of Audiology, American Academy of Otolaryngology-Head and Neck Surgery, American Cochlear Implant Alliance, American Speech-Language-Hearing Association, Coalition to End Social Isolation & Loneliness, the Ear Community, Healthy Hearing, Hearing Health Foundation, Hearing Loss Association of America, International Hearing Society, National Council on Aging, National Grange, and Retire Safe.
www.hearing.org
www.betterhearing.org
www.fda.gov








