Over-the-counter (OTC) hearing aids are a new product category that consumers will soon be able to buy directly from brands that we all associate with consumer lifestyle products, such as the recently announced Bose SoundControl product. Intended for adults who believe they have mild-to-moderate hearing loss, OTC hearing aids will be regulated as medical devices by the US Food and Drug Administration (FDA). There is also an existing category of assisted hearing devices, personal sound amplification products (PSAPs), bridging gaps between medical hearing aids and consumer earbuds.
The next generation of these OTC and direct-to-consumer devices must include not only legacy hearing aid features but also TWS consumer earbud features, including Bluetooth and possibly active noise cancellation (ANC). Several challenges are presented when trying to combine the compact, consumer-friendly form factor of TWS with hearing assistance features into a single product.
Challenge 1 – Form factor
The first challenge is finding balance to achieve that small, in-ear TWS form factor while also delivering operation times somewhere in between TWS and a medical-grade hearing aid. Fitting large, thick dynamic driver (DD) transducers together with larger batteries required for extended operation time is difficult. Also challenging is managing magnetic sources in close proximity to RF Bluetooth antennas.
With medical-grade hearing aids, audiologists have the luxury to mix and match a variety of Balanced Armature (BA) drivers to deliver a unique frequency response tuned to the specific hearing loss profile of an individual consumer. This is very much a custom product. For OTC, there is no such structure. A single driver must be flexible enough to adapt to the hearing needs of millions of unique consumers. OTC products include a self-assessment app that runs the consumer through a series of tests to identify with which frequencies the consumer requires assistance. Then a custom equalization (EQ) profile is applied via DSP to the transducer to deliver the desired sound profile.
For this consumer-driven method to work, the OTC vendor requires a speaker/driver with a sufficiently flexible frequency response to adapt to many combinations of low, mid, and high frequency hearing loss. This can be challenging for a single-configuration (single SKU) product, where one transducer must have the flexibility to serve all hearing profile needs.
Further, once the product depends on DSP and parametric EQ for tuning, developers must also care about driver-to-driver variations in frequency response. A wide variation (+/-3dB or more for typical coil-based transducers) will make it difficult for the EQ to always have the desired effect. This can be overcome with driver matching and calibration at assembly, at the expense of test time and cost.
Challenge 3 – Comfort Without Listening Fatigue
Finally, OTC product designers need to achieve a balance in listening comfort. An OTC product may not be worn for 18 hours like a medical-grade hearing aid, but wear may regularly exceed that of the average TWS use case. Two key measures of long-wear comfort include ear canal pressure mitigation and the overall weight of the product as it rests inside or hangs on the ear.

Just as integrated circuits were a key building block to the modern hearing aid, there are new components that will enable augmented hearing in TWS form factors. What is interesting is that again it is an integrated circuit... specifically, a new class of microspeakers now available that may enable both easier earbud design and manufacturing while at the same time enabling broad, wide-bandwidth frequency response tuning to any type of hearing assistance need all from a single, non-custom, mass-manufactured earbud.
xMEMS Labs, a California startup with an experienced team of microelectromechanical systems (MEMS) specialists and audio veterans from Apple, Knowles, InvenSense, and Forte Media, is targeting over-the-counter hearing augmentation enhanced earphones in addition to the traditional consumer TWS market.
MEMS microspeakers have trailed behind MEMS microphone commercialization by two decades due to materials and fabrication challenges, but now, with the emergence of thin-film piezo materials, MEMS microspeakers can actuate more quickly and with greater precision than traditional coil-based transducers, enabling production of high-fidelity audio. Today, devices from xMEMS deliver a combination of audio fidelity, size, and speaker-to-speaker uniformity that is not possible with traditional voice coil (DD or BA) approaches.
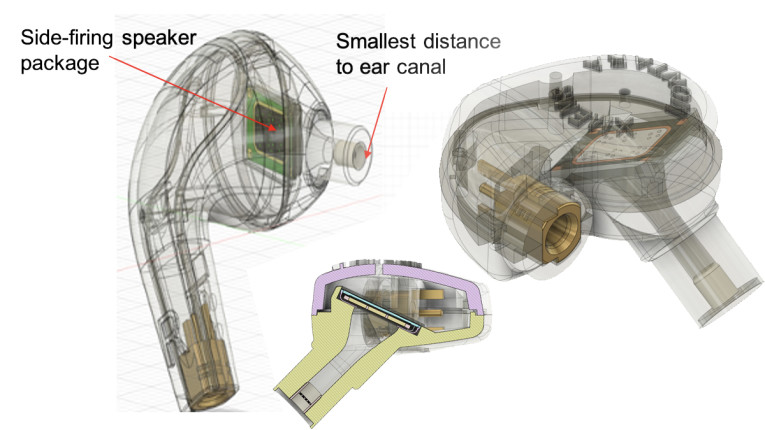
xMEMS introduced its Montara microspeaker in July 2020. The entire speaker (actuator and diaphragm/membrane) is manufactured in a high-precision, highly consistent monolithic silicon process (<10 layers). The net result is a very thin slim speaker (1mm thin) that consumes approximately 50mm3 area and weighs less than 80mg. Future derivatives will achieve sub-30mm3 area, enabling Receiver-in-Canal (RIC) implementations. But it is not just the fabrication of the transducers, but the promise of automated pick-and-place of MEMS speakers for surface-mount technology (SMT) board stuffing. While SMT circuit board construction is not used for most prescribed hearing aids, multi-layer SMT is common for TWS devices. For placement flexibility within the earbud housing, Montara offers two SMT-ready package options: top-firing and side firing.
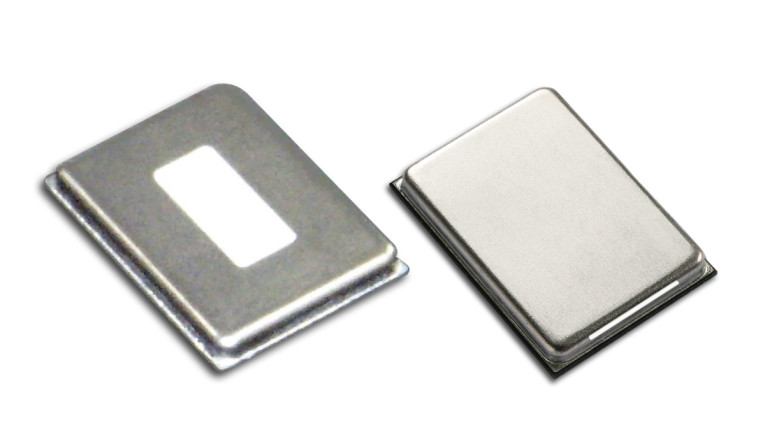
And MEMS speakers are voltage-driven, capacitive devices and are extremely power-efficient. Opportunities exist in the future to pair them with amplifiers capable of energy recycling to further extend operation time beyond what is possible today from even the most efficient BA.
Finally, the acoustic uniformity of MEMS earphones enables viable self-assessment tuning. Fabricated using a monolithic MEMS semiconductor process, xMEMS' Montara creates unprecedented transducer-to-transducer consistency (+/- 1.5 dB variation), which eliminates calibration and driver matching common with coil-based drivers. The xMEMS speaker utilizes a silicon diaphragm that is rigid while damped, ideal for OTC occluded hearing augmentation applications.
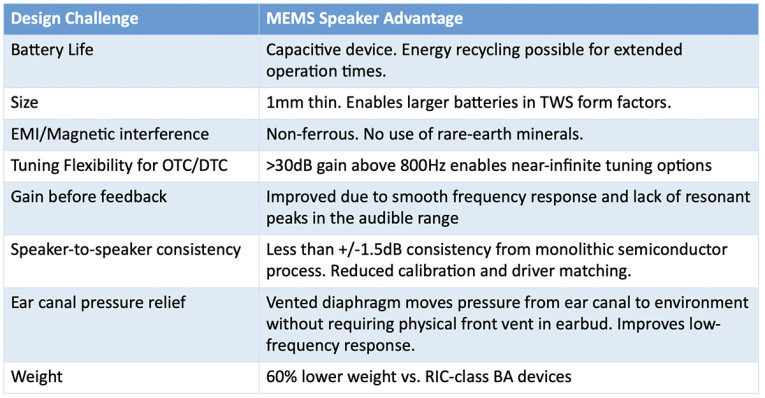
As highlighted in the Figure below, the linear response without resonance of the xMEMS transducer rises smoothly above 800Hz to beyond 20kHz, to achieve near infinite frequency response curve configurability when using equalization. Up to 30dB of gain is available in the upper octaves to aid high frequency hearing loss scenarios and to support improved speech intelligibility, presence, and clarity. An EQ setting, such as a Harman curve, can be applied to the xMEMS solution and be further converted into biquad coefficient sets, the more accessible format for DSP, to also deliver the similar frequency response.
Most important, the smooth phase, fast transient settling time, and extra upper octave amplitude allow for a significant increase in gain and bandwidth without feedback compared to balanced armature transducers or any of the 6mm diameter dynamic earphone drivers. Specifically, the resonance-free response of the xMEMS earphone speaker enables the self-assessment remedial compensation to work better than BAs – as hearing-aid fitters know all too well the skill needed to precisely tune BAs for clients.
Some OTC hearing augmentation earphones are targeting both higher speech intelligibility, but also enhanced music and movie listening experiences. As the xMEMS device is free of the high Q resonance of the BA drivers, hearing compensation well past 8kHz is possible. Beyond enhanced speech augmentation, the device's fidelity offers unique attributes of musicality to hearing aids.
From the user’s perspective, listening fatigue may be the most perceived benefit of xMEMS microspeakers due to the integrated venting. Montara’s silicon speaker membrane (equivalent to diaphragm) is vented with microscopic slits so a pressure differential pathway exists between front volume (ear canal) and the back volume of the speaker without negatively impacting low frequency response. The back-volume housing is often open to the outside with a vent so a secondary vent for over-pressure protection may not be required.
And the fact that MEMS microspeakers eliminate the coil and large magnet of traditional dynamic drivers leads to a much lighter earbud (60% lighter than a typical RIC BA receiver), which will reduce strain on the ear and improve long-term comfort.
In summary, MEMS microspeakers promise to break the last remaining barriers to shrinking the OTC hearing augmentation devices, increasing comfort, while adding features and extending battery life. Most significant are improved speech intelligibility, presence, and higher gain before feedback, conferred from the extended and smoother mid/high frequency response. Additionally, the acoustic uniformity of MEMS earphones enables viable self-assessment tuning. xMEMS devices and evaluation kits are sampling to manufacturers now, with mass production already scheduled in 2021.