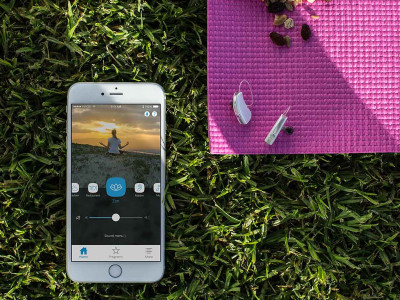
ZVOX Announces VoiceBud VB20 Full-Featured Over-the-Counter Voice-Enhancing Hearing Device
ZVOX Audio announced the availability of its VoiceBud V20, the company's first-ever personal audio device. As the company believes the category will be booming in the next 18 months as more companies enter the over-the-counter (OTC) personal sound amplification product business, ZVOX designed a very affordable ($299) device equipped with dual-microphone NoiseBlocker technology and smartphone app control using high-performance American-made electronics components.