
The proposed rule amends existing rules that apply to hearing aids for consistency with the new OTC category, defined as "certain air-conduction hearing aids intended for adults age 18 and older who have perceived mild to moderate hearing loss," while "Hearing aids for severe hearing loss or for users younger than age 18 would be prescription devices."
There is a lot to take in with those guidelines, containing proposals to ensure patient safety, including volume limits for OTC hearing aids, device performance and design requirements, including latency limits, as well as a requirement to limit the insertion depth of the device and labeling requirements. The updated FDA draft guidance, "Regulatory Requirements for Hearing Aid Devices and Personal Sound Amplification Products (PSAPs)" contains a lot of useful information that will now be discussed in detail by the companies that are certain to be more involved in the topic, which are obviously the hearing-aid and audiology companies, most of which these days have already invested in separate consumer electronics divisions and brands.
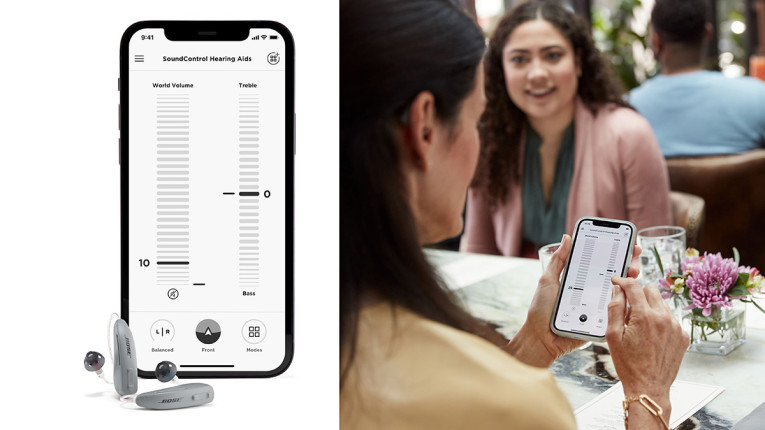
I know that many audio manufacturers see this as an opportunity and I know that a lot of large, medium, and small technology companies have been working for years to pursue the "OTC hearing aids" opportunity. Most of those companies are not familiar with the complexities of global regulatory environments for medical and health products, which makes those investments look somewhat displaced and reckless. In the past years, I have seen multiple examples of companies determined to pursue the health impact of hearing loss, with more or less altruistic goals. More recently, that level of activity increased exponentially, particularly since the disclosure by Bose of its commitment to the OTC segment, and even more so after Apple revealed the hearing assistance possibilities of its popular AirPods.
But the more I look into how this is evolving, and the more I look at the product evolution and announcements from players on the medical hearing-aid and consumer technology sides, the more it seems apparent to me that there are two possible, independent strategies - both beneficial, rewarding, and profitable.
At least for now, you can't have both. In my opinion, most audio manufacturers are better off by not pursuing the FDA-approved OTC market. Doing so will simply hinder critical experimentation and innovation. The consumer technology approach needs to be constantly adjustable, dynamic, fast, and bold… sometimes crazy. A regulated, medical approach also needs intensive experimentation and innovation, but under specific, highly controlled testing scenarios, outside the realm of consumer discretion.
And there's a very good reason for that, with useful references in the relationship between ophthalmology, vision care, prescription lenses, and the eyewear industry. One that apparently the consumer electronics companies (and in particular the technology giants such as Google or Facebook) don't seem to understand when they pursue "smart glasses" that do everything except help people to see.

That Could Be Smart
Anytime a consumer receives a prescription to buy lenses to help him see better, he also buys frames, helping to maintain an "healthy" business for the eyewear industry. The average manufacturing cost of frames is $25 and retail upward of $500 for the final product. Prescriptions help keep the eyewear industry in business. For a bit of perspective, contact lenses and OTC reading glasses are less than 20% of the overall eyeglass market. The global contact lenses market size was valued at $12.79 billion USD in 2019 (www.grandviewresearch.com). Eyewear frames and spectacles are valued at $52 USD billion in the same year.
If I were to pursue research aiming to make glasses smarter, I would approach tracking our eyes, measuring vision ability at any given moment, and automatically adjusting the lenses to what we are looking at, close, mid, or long distance - contemplating the whole field, or reading fine print in a screen. No one needs another screen in their prescription lenses, and I certainly don't need a camera or Bluetooth audio on my glasses that are intended to help my eyes focus and instead could cause me to see images that appear blurry. As everyone who "needs" to wear prescription lenses will confirm, we want glasses to disappear. Everything else, is just a nuisance.
Consumer electronic companies designing hearing enhancement features in true wireless earbuds are better off staying away from the OTC "potential" market. A bit like Nuheara, which has created a direct-to-consumer model, or Apple with its accessibility features for the AirPods that have been effectively supporting users daily. Both companies are just starting to understand what is effective and what isn't, where the challenges are, and how to evolve the next iterations.
Hearing enhancement, including simple sound personalization profiles, can be beneficial to users suffering from mild-to-moderate hearing loss. But by not making declared promises in that sense, and by not trying to be replacements of hearings aids or even PSAP devices, these companies gain the much required time to allow a large number of consumers to become familiar with the technology and discover the technology possibilities, spreading its benefits to other potential users in result. It’s already happening.

In the same way, the medical hearing-aid-focused companies will also have the encouragement to match as much as possible a class of products that consumers already know and appreciate, removing the stigma of wearing something that could flag a disability. That has also already happened with the fast adoption of consumer true wireless earbuds, and hearing-aids are now adopting similar form-factors because of it.
I still remember my reaction of surprise (and some disgust) when I saw the AirPods design with its white prong for the first time. I still don't like to see the white things protruding from people's ears (or my own), but I am no longer surprised and I can see that it became an accepted form-factor - which now everyone is copying. And as a regular AirPods user on Skype/Zoom calls, I have learned to recognize the benefits of having a microphone that works better thanks to being closer to the mouth.
But there are more important reasons for consumer electronics companies to stay away from the OTC label and regulated market approach. The same hearing enhancement features that are beneficial to people with mild-to-moderate hearing loss, are also recognized and appreciated by everyone else. It's interesting to discuss how differences in hearing abilities and different hearing profiles help explain the popularity of sound personalization, namely left/right unbalances. In fact, there is an important difference with hearables, which is at what point are they no longer just compensating for hearing deficiencies and are instead enhancing our hearing.
As our colleague Brent Butherworth - who doesn't have hearing loss but still has been reviewing hearables and hearing aids for audioXpress - has quickly discovered, for someone familiar with audio frequency bands, response curves, and how these devices work, it becomes irresistible to tweak the features to gain "super-powers." Enter hearing augmentation, which is the next big frontier for hearables in general — and that's a consumer electronics application and not medical-related.

Hearing aid companies are well-ahead in this research and they understand this better than anyone else. They were just lacking in obvious things, such as Bluetooth and other mass market technologies that were the enablers of true wireless earbuds - and they are now adjusting quickly. And with the upcoming Bluetooth LE Audio technology, they will have another significantly solid foundation to create market leading hearing-aid solutions to bring to market.
The questions remaining are: Will audiology companies be happy to remain in their medical space, or will they pursue the mine-field of OTC labels? Or even, will they prefer to leverage their strengths to go after the market opportunity that is TWS and all the potential applications of hearables and hearing augmentation for business and many other applications? Or maybe even, will they create consumer "platforms" that can accommodate medical-grade hearing-aid technology (and new forms of subscription-based services), similar to the lenses/eyewear frames model? I believe the answer has already been given by recent moves such as the Sonova acquisition of Sennheiser's consumer products division, and GN Group's strategy with Jabra, ReSound, and BlueParrott.
For the January 2022 edition of audioXpress, Brent Butterworth wrote another key review of a product that was designed and long-advertised as being suitable to enter the opportunity that was the OTC space. That review was supposed to have been published in an earlier edition of the magazine, but our reviewer was caught by surprise with the announcement that the company (based in Japan) had decided to pause sales in North America because even though it’s done a lot to make its product user-friendly and easy to set up, it also found “many customers still need help with the product.” The decision was taken to stop for now, build up customer support operations, including a 24-hour call center for US customers, and relaunch the product later. “Having the call centers will also give us a lot more feedback from customers, which will help us improve the product,” they stated.
This company is Olive Union, which launched its second-generation Olive Pro ($299), in December 2020. "Although the Olive Pro can’t be programmed by an audiologist, it’s possible that a sophisticated user could take the results of an audiogram and use those with the EQ to get a more accurate compensation for their hearing loss," Brent wrote, characterizing the issue.
Hearing Augmentation
Why does hearing augmentation have more consumer appeal and market potential than hearing enhancement? Ask Apple.
Apple has seen that opportunity a long time ago. In fact the massive success of the Apple Watch (which sells more products than the combined Swiss watch industry combined) is now visibly related with its enormous health and wellness potential - even though it was originally introduced and promoted as a technology/lifestyle product.
The key word here is "health," which is something that all consumers worry and care about, and are willing to pay for reasonably well. If by wearing a watch, people gain insights into their well-being and avoid finding out too late about a medical condition, the Watch is a must-have. Of course, Apple is conducting all sorts of research in medical applications for its built-in biometrics - and anonymously collected data as it largely advertises - but they know too well that they need to stay away from pursuing those goals until the scientific and medical communities themselves award that recognition, which in turn would make it worth pursuing certifications.

With hearing augmentation, we are facing a related but slightly different concept that is directly connected to the wider concept of augmented reality. Facebook apparently is determined to pursue putting goggles on people to convince them that escaping for an alternate gaming reality called "Metaverse" could be the ultimate goal for humanity. A lot is being discussed about this revamp of Second-Life (see Wikipedia if you don't remember).
In comparison, I've seen very few commentary to the amazing power that is now available with the recent operating system updates that Apple released, filled with the best examples ever of augmented reality. Some, like the possibility to digitize 3D objects with the iPhone camera, are just the start - the APIs are already generating stunning new apps. But the benefits of just taking a photo and being able to digitize a phone number and immediately making a call, or pointing the camera at a newspaper article and copying a large chunk of clean text - ready to paste into social media, or a research paper, is just too big to ignore.
Likewise, my wife has been fascinated with the amount of information she can gather from pointing the camera at birds, flowers, and trees, immediately getting the names of the species and extra information. Or the way Apple Maps now displays 3D street names and arrows showing us the way around the city (available only in a few countries for now). All this, totally integrated among devices and available at the operating system level. All too good not to recommend.
Similarly, hearing augmentation is the ability to apply that concept further and automatically have an optimized hearing filter that "augments" the things that are important - such as speech from the person we are looking at - while reducing or eliminating street noises nearby. As Apple has perfected, all those features can work better when combining a series of input sensors that start with the smartphone, the Watch and its powerful activity sensors, and the minuscule earbuds in our ears to process and feed exactly the correct sound signal. How do the AirPods know which person in a room is the one we want to hear? Apple apparently is working on sensors to read eye movements, and that would be a reason to wear “smart glasses” - as long as we can still change the prescription lenses.
There's a lot to do and improve to make audio augmentation useful for everyone. But the same could be said of implementing text recognition from a photo. I remember testing an app for that years ago, and I was extremely impressed. Now, I have it on all devices, all the time. What people will do with it will vary greatly, but the best attitude is to be cautious and allow the time and the opportunity for users to get familiar with a certain technology. A similar approach is required for hearables to evolve.








